“For the millions of patients fighting for a chance to try, we must do what we can to help them. Right to Try is not a cure, and it’s not a miracle, but it is a way to hold on to hope,” Johnson said.

“Time after time we were just continually disappointed by the lack of progress being made in this area. So hope starts to fade. Having a bill like this introduced, giving somebody an opportunity for hope, is something that’s incredibly important.” Tim Wendler

Click Here to learn more about Trickett Wendler's story
“This right to try legislation, which basically gives patients the right to medications that can save their life.” – Laura McLinn about her son, Jordan, 7
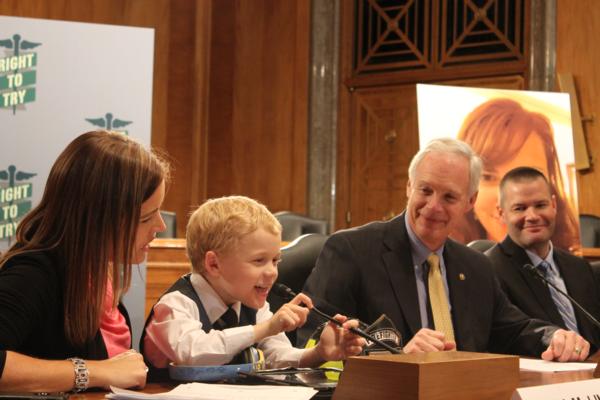
Click here to learn more about Laura and Jordan McLinn’s story.
“This is providing hope for us, for a better life. There shouldn’t be anybody standing in the way.” Matt Bellina

Click here to learn more about Matt Bellina’s story.